How Does Rhodium Plating Work? An Illustrated Guide to Getting Rings Redipped
When you’re getting rings redipped, you might be wondering, how does rhodium plating work? Find out with this illustrated guide!
This article was originally published on a WordPress blog that I’ve retired in favor of Substack. Thanks for reading!
Getting rings rhodium plated is a fairly straightforward process that most jewelers have the right tools and equipment to do. When you’re getting rings redipped, you might be wondering, how does rhodium plating work? After you hand over those sterling silver or gold rings in need of a fresh new rhodium finish, what happens to them?
I've created this illustrated guide to show you the basic process of getting rings rhodium plated.
Rhodium plating basics
Rhodium-plated rings (and gold-plated rings, too) are coated in a layer of metal using the electroplating process.
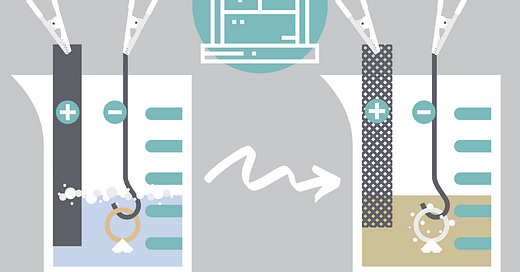
In the most simple terms, your jeweler will prepare and dip your ring into a series of electrically charged solutions. The ring and the solutions are each given their electric charge using a machine called a rectifier.
Before the plating process begins, the jeweler will suspend the ring from a small handling wire. This is usually made of gold, platinum, or stainless steel. They’ll connect that wire to the cathode (-) to pass a negative charge through your ring.
Your ring will be primed in a couple of different solutions to start. Then, once it gets to the rhodium solution, the jeweler will clamp a strip of metal to the anode (+), giving it a positive charge. They’ll place that anodized piece of metal into the solution, sending a positive charge throughout the liquid and the rhodium particles.
Since opposites attract, the positively charged rhodium particles will latch onto your negatively charged ring. Through an electrochemical reaction, a gleaming coat of white metal will be deposited all over the surface of the ring.
But before that happens, your ring needs to bathe in a few other beakers first. Let’s take a closer look at each solution used in the rhodium plating process:
Solution 1: Electrocleaner
The first beaker will contain an electrocleaning solution, which is made up of water plus an alkaline-based degreasing product. This cleans off the surface of the ring and removes any residue, oils, or buildup.
The jeweler will heat up the electrocleaning solution and insert a stainless steel anode to send a positive electrical current through the solution.
When the negatively charged ring gets dipped into the positively charged solution, hydrogen bubbles will rise up to the top of the liquid as the ring gets electrochemically cleaned.
After a short period of time (somewhere between 30 seconds and a couple of minutes), the jeweler will dunk the ring into a beaker of fresh water to rinse it off.
Solution 2: Acid dip neutralizer
The second solution is an acid dip solution which contains a corrosive acid combined with water.
The jeweler will turn off the voltage from the rectifier so the ring is no longer negatively charged. They'll then lower the ring into this neutralizing acid dip to stop the effects of the electrocleaning solution.
After a short time in the acid dip, the jeweler will conclude this step with another fresh water rinse. Now, your ring is primed for the electroplating process!
Solution 3: Rhodium solution
The last solution your jeweler will use contains particles of rhodium. The jeweler will heat up this solution and drop in an anodized piece of platinized titanium. This piece of metal delivers a positive charge to the solution and charges all the rhodium particles floating around in it.
Once the jeweler sets the voltage and lowers the negatively charged ring into this liquid, hydrogen bubbles will form on the ring’s surface, indicating that the electrochemical process is underway.
Particles of positively-charged rhodium will be gradually deposited onto the negatively charged ring. After a little while, all the metal parts of your ring will be left with a thin and even coat of rhodium.
Final steps
The jeweler will swirl your rhodium-plated ring around in another fresh water rinse. Finally, they’ll neutralize it once again in the acid dip solution and follow up with one last fresh water rinse.
In just a few simple steps, your sterling silver or gold ring will be completely transformed.
If you're looking for more information, here's a great video demonstration showing the process of getting rings redipped in rhodium.
Now that you know how rhodium plating works, how do you feel about getting rings redipped? I personally love the cool electrochemical process!
Illustrations by Jess Barker